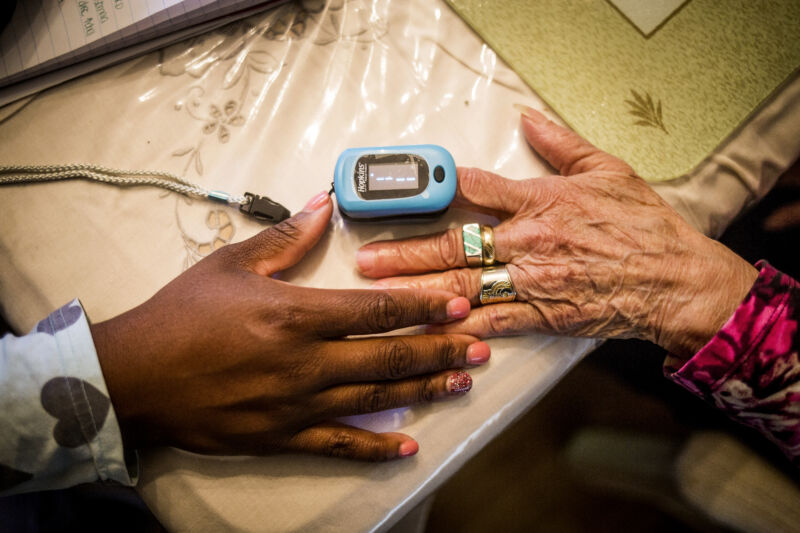
Enlarge / A nurse makes use of a pulse oximeter on a affected person in Plainfield, New Jersey, on October 26, 2016. (credit score: Getty | Bloomberg)
For years, research have discovered racial bias in frequent oxygen measuring gadgets known as pulse oximeters, in addition to alarming risks from inaccurate blood oxygen measurements in dark-skinned sufferers. Now, the US Meals and Drug Administration is summoning its professional advisors to evaluate the problematic gadgets and take into account new suggestions and regulatory actions.
The FDA introduced Thursday that its advisory committee—the Anesthesiology and Respiratory Remedy Gadgets Panel (ARTDP)—would convene on November 1 to debate pulse oximeters. Till then, the company renewed emphasis on the security warning it issued in February 2021, which famous that the ever-present gadgets “could also be much less correct in folks with darkish pores and skin pigmentation.”
That warning intently adopted a examine from December 2020 that highlighted the racial bias of pulse oximeters amid the COVID-19 pandemic. The worldwide unfold of a respiratory illness with a trademark symptom of respiratory problem despatched pulse oximeter utilization hovering—elevating the issue of racial disparities. The 2020 examine—led by researchers in Michigan and printed within the New England Journal of Drugs—discovered that pulse oximeters have been practically 3 times extra prone to miss dangerously low blood oxygen ranges (hypoxemia) in Black sufferers in contrast with white sufferers.
Learn 5 remaining paragraphs | Feedback